Search results
Search for "halide abstraction" in Full Text gives 9 result(s) in Beilstein Journal of Organic Chemistry.
Halides as versatile anions in asymmetric anion-binding organocatalysis
Beilstein J. Org. Chem. 2021, 17, 2270–2286, doi:10.3762/bjoc.17.145
- proton of the enolizable β-ketoester 49 and thus activating the nucleophilic species. This enolate then adds to the cationic substrate from in situ upon halide abstraction of α-chloro amino acid derivatives 48 by the thiourea moiety of the bifunctional catalyst (Scheme 10c, key intermediate), leading to
- macrocycle 81 (Scheme 17a) [78]. Compared to bis-thiourea 80, the higher rigidity in the macrocycle 81 not only enforces halide abstraction significantly, but also allowed for a better control of the stereoselectivity in the glycosylation of glycosyl halides 78 with a variety of coupling partners. In this
Methodologies for the synthesis of quaternary carbon centers via hydroalkylation of unactivated olefins: twenty years of advances
Beilstein J. Org. Chem. 2021, 17, 1565–1590, doi:10.3762/bjoc.17.112
- changing the previously optimized mild reaction conditions (Scheme 6). Only traces of 10 were observed in the absence of one of the metal salts; this excluded the participation of the silver salt in the olefin activation but highlighted its usual role in the activation of the gold catalyst, namely halide
- abstraction, to form a gold complex with a less coordinating ligand that serves as the active catalyst [39]. Following their initial work, the Li group investigated the use of silver triflate as the sole catalyst in the olefin hydroalkylation [40]. In their previous work, they had reported that neither the
Fluorinated azobenzenes as supramolecular halogen-bonding building blocks
Beilstein J. Org. Chem. 2019, 15, 2013–2019, doi:10.3762/bjoc.15.197
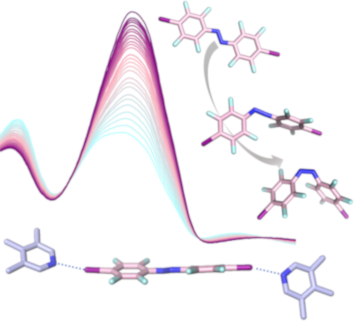
- ][22][23][24]. Huber and co-workers demonstrated the activation of a carbonyl group by halogen bonding, and successfully applied this concept to catalysts for Michael addition reactions [25] and also employed neutral [26], and hypervalent iodolium derivatives as activators in a halide abstraction
Enantioselective Diels–Alder reaction of anthracene by chiral tritylium catalysis
Beilstein J. Org. Chem. 2019, 15, 1304–1312, doi:10.3762/bjoc.15.129
- investigated in Lewis acid catalysis over the past decades, a chiral counter anion [34][35] with metal elements as the central atom, however, was seldom reported. Typically, the tritylium salts with weakly coordinating anions can be synthesized through a simple halide abstraction from the trityl halide in the
Synthesis of dihydroquinazolines from 2-aminobenzylamine: N3-aryl derivatives with electron-withdrawing groups
Beilstein J. Org. Chem. 2018, 14, 2510–2519, doi:10.3762/bjoc.14.227
- generated by Lewis acid-promoted halide abstraction from imidoyl chlorides or by alkylation of nitriles. A nucleophilic attack on such species followed by cyclization provides access to a variety of heterocycles. Intramolecular reactions of in situ generated nitrilium ions leading to heterocyclic compounds
A modular approach to neutral P,N-ligands: synthesis and coordination chemistry
Beilstein J. Org. Chem. 2016, 12, 846–853, doi:10.3762/bjoc.12.83
- similarities. Bond lengths and bond angles of the chelate ring agree well, underlining that halide abstraction is effectively compensated by the bridging chlorides (Table 3). Significant structural deviations are only observed for the Pd–Cl bonds, where significantly longer bonds were found for the cationic
Evidencing an inner-sphere mechanism for NHC-Au(I)-catalyzed carbene-transfer reactions from ethyl diazoacetate
Beilstein J. Org. Chem. 2015, 11, 2254–2260, doi:10.3762/bjoc.11.245
- precursors were dissolved in the neat substrate (5 mL) and stirred for 30 min to ensure halide abstraction prior to EDA addition. The experiments have been carried out using a flask connected to a pressure gauge that provides the variation in the increase of the internal pressure (see Experimental). Figure 1
True and masked three-coordinate T-shaped platinum(II) intermediates
Beilstein J. Org. Chem. 2013, 9, 1352–1382, doi:10.3762/bjoc.9.153
- al. successfully characterized a true 14-electron T-shaped Pt(II) boryl complex T2a by means of X-ray studies [20]. By halide abstraction, the cationic trans-[Pt(B(Fc)Br)(PCy3)2]+ T2a (Fc = ferrocenyl; Cy = cyclohexyl) could be obtained, in which the boryl ligand is located trans to the empty site
The role of silver additives in gold-mediated C–H functionalisation
Beilstein J. Org. Chem. 2011, 7, 892–896, doi:10.3762/bjoc.7.102
- functionalisation of aromatic C–H bonds. Doubt is cast on the commonly cited route of halide abstraction from gold and evidence of substrate activation is given. Keywords: C–H functionalisation; gold catalyst; halide abstraction; N-heterocyclic carbene; silver salt; substrate activation; Introduction The use of














































































































































































